Agilent provides xCELLigence impedance-based, label-free, real time cell analysis system and NovoCyte flow cytometers.
Automated microscopy and Spatial Proteomics
Real-time, label free cell analysis
Nano and micro particle analysis
Accelerate to discover
Related topics
Discover the Celloger live-cell analyzers : miniaturized and powerful tools for every Biologist

Apr 5, 2024
Celloger live-cell analyzers, image and analyze your cells in real-time, inside your incubator. With its exceptional...
The chicken chorioallantoic membrane as a low-cost, high-throughput model for cancer imaging

Apr 4, 2024
Here, we assessed the chicken chorioallantoic membrane (CAM) as an alternative to mice for preclinical cancer imaging...
A microthrombus-driven fixed-point cleaved nanosystem for preventing post-thrombolysis recurrence

Apr 3, 2024
A thrombin-responsive and fixed-point cleaved Fu@pep-CLipo was developed for highly efficient and precise thrombolysis...
Webinar: Multimodal Assessment of Hypoxia in Tumors: From the Lab to the Clinic

Apr 2, 2024
25 April 2024, 4PM CET
This webinar will be of interest to multiple profiles in the community of biomedical...
Multiplexed tissue imaging using the Orion platform to reveal the Spatial Biology of Cancer

Mar 27, 2024
In this webinar Prof. Sandro Santagata, will reveal how Orion high-plex imaging and the use of this data, is valuable...
Cytek SpectroFlo Software Version 3.3 has been released!

Mar 25, 2024
SpectroFlo Software Version 3.3 has been released, enabling new assays with SpectroFlo Software version 3.3, including...
A 19-color single-tube Full Spectrum Flow Cytometry for the detection of Acute Myeloid Leukemia

Mar 13, 2024
This recent publication in Cytometry Part A describes the development and comprehensive workflow of a single-tube,...
18F-labeled somatostatin analogs for somatostatin receptors (SSTRs) targeted PET imaging of NETs

Mar 11, 2024
A novel 18F-radiolabeled somatostatin analogue, [Al18F]NODA-MPAA-HTA, was synthesized and evaluated for positron...
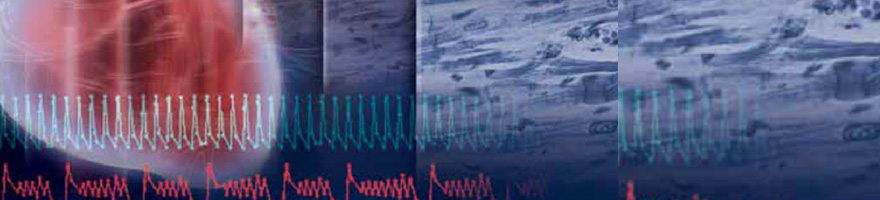
Mar 22, 2016
Over the last two decades, a number of blockbuster drugs have been withdrawn or have incurred safety warnings by regulatory agencies due to adverse cardiac effects. In addition, lead compounds or drug candidates are frequently terminated at late stages of drug development due to cardiac safety concerns. Both of these factors can significantly impact the overall cost of drug discovery; consequently, pharmaceutical companies and regulatory agencies have implemented procedures to address these issues.
Most, if not all, in vitro assay systems for cardiac safety are designed to screen for surrogates of arrhythmia, such as hERG channel interaction, rather than arrhythmia itself. Assays designed to screen for compounds that may affect repolarization and induce arrhythmia in the context of the whole heart or heart tissue are not implemented until much later in drug development. These include sophisticated, technically demanding, lowthroughput, and costly procedures such as the Purkinje fiber assay, ventricular wedge assay, and the Langendorff whole heart assay, or telemetry experiments in live and anesthetized animals. The field of preclinical cardiac safety can certainly benefit from an assay system that allows for integrated assessment of compound action on ion channel and non-ion channel targets involved in cardiac excitation-contraction coupling.
The RTCA Cardio Instrument in conjunction with iCell Cardiomyocytes comprises an assay system providing integrated assessment of compound action on multiple targets involved in heart function. This assay system can sensitively and quantitatively detect the effect of compounds on the major ion channels involved in heart function, namely calcium, sodium, and potassium channels. Another major advantage of the assay system is the time resolution. The RTCA Cardio Instrument has a data acquisition rate of 12.5 ms per well of a 96-well plate and can be used simultaneously to monitor acute and chronic drug effects up to days and weeks. The utility of the time-dependent monitoring of compound action on cardiomyocytes can be demonstrated by testing compounds that acutely and directly block hERG channels (E4031, cisapride, etc.) and compounds that interfere with protein trafficking (pentamidine) in a sub-chronic manner. The RTCA Cardio Instrument is able to detect the effects of these compounds on the beating rate and duration of iCell Cardiomyocytes.
The xCELLigence System RTCA Cardio Instrument, in conjunction with iCell Cardiomyocytes, represents a physiologically relevant and predictive assay system for preclinical cardio-safety assessment of lead compounds. The features of this assay system, including time resolution, dynamic monitoring of mechanical beating activity of cardiomyocytes, and 96-well throughput, will surely provide additional mechanistic and toxicity information for compound action on the heart.
Related technologies: Real-time, label free cell analysis
Brand profile
Agilent provides xCELLigence impedance-based, label-free, real time cell analysis system and NovoCyte flow cytometers.
More info at:
www.aceabio.com